A humanised mouse can accelerate drug discovery
Preclinical demonstration of the efficacy of a drug in a mouse model of disease is a key step in drug discovery. However, this step can be impeded by the presence of mouse-specific pathways of drug metabolism. A team comprising researchers at the University of Dundee, including Roland Wolf, a member of the Network’s Degron Tagging Cluster, and collaborators at the GlaxoSmithKline Tres Cantos Open Lab have published a paper in PNAS that demonstrates how a mouse line extensively humanised for genes involved in drug metabolism can be used to accelerate infectious disease drug discovery and development.
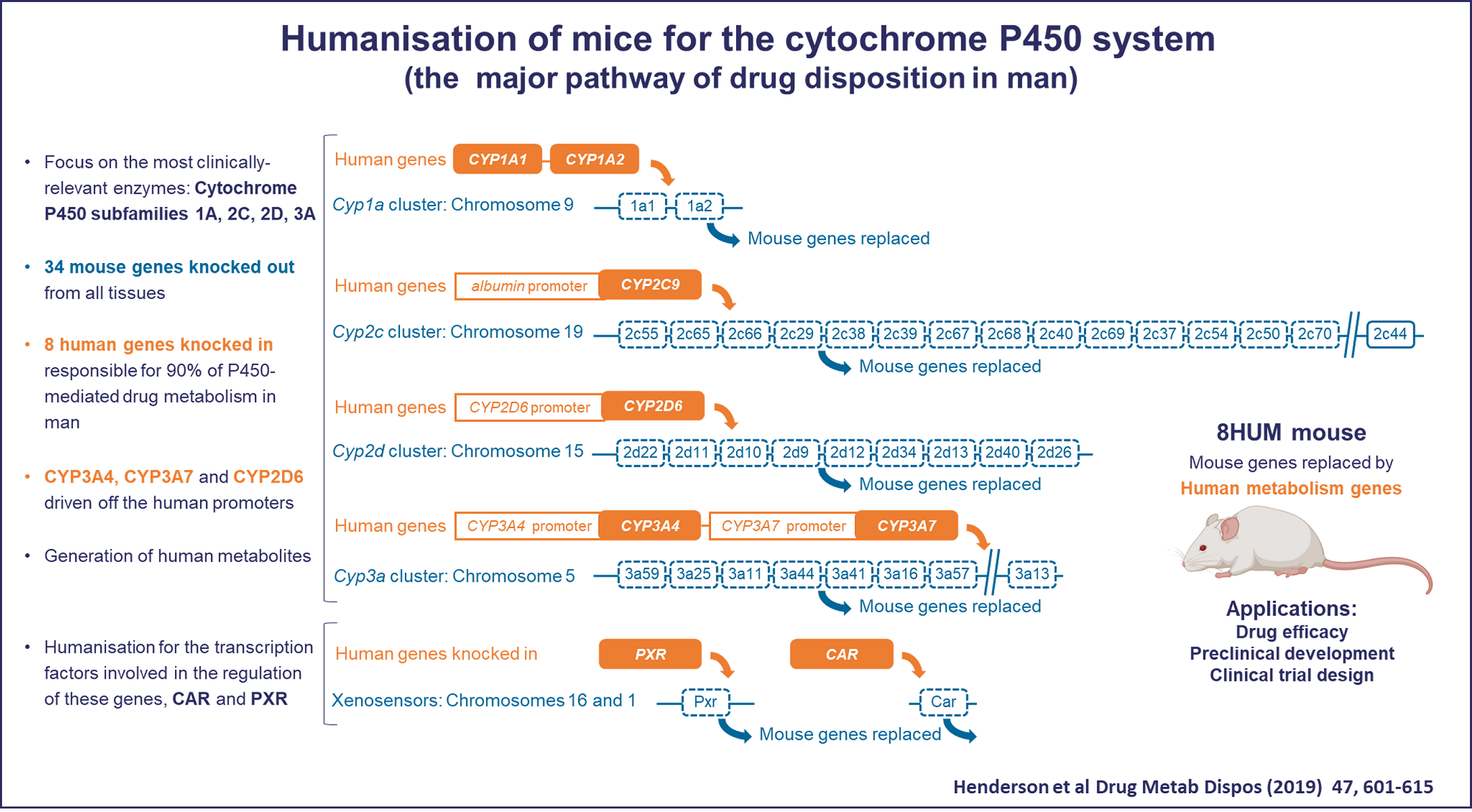
The cytochrome P450 (CYP) superfamily is responsible for catalysing 50 –70% of the biochemical processes required to metabolise and eliminate the small molecule drugs that we take to treat a wide variety of diseases. The majority of this activity is performed in humans by only eight proteins within four CYP subfamilies. By contrast, mice have 34 genes within these subfamilies and there are major differences between mouse and man in the way in which drugs are metabolised and, invariably, mice metabolise drugs much more rapidly than humans. This creates a major issue in demonstrating drug efficacy in mouse models of disease and necessitates the development of mouse lines that better model drug metabolism alongside modelling the disease of interest.
To address this issue, Roland Wolf and colleagues at Dundee worked with CXR Biosciences and Taconic Biosciences to create an extensively humanised mouse line, “8HUM”, in which the genes for 33 mouse CYPs, together with two transcription factors (Pxrand Car) were deleted and replaced with just 6 human CYPs responsible for 90% of P450-mediated drug metabolism in man (CYP1A1, CYP1A2, CYP2C9, CYP2D6, CYP3A4, and CYP3A7) and the human transcription factors PXR and CAR. They previously showed that levels of hepatic CYP expression in this model are highly similar to those in humans and that co-administration of a set of drugs with inducers of different CYP proteins showed comparable pharmacokinetics to those seen in humans, demonstrating the broad potential of this model for drug discovery.
In their new paper, involving Professor Wolf together with Professor Kevin Read and Dr Kenneth MacLeod of the University of Dundee Drug Discovery Unit, the 8HUM model has now been tested in an industry-standard drug discovery workflow, using a panel of approved medicines used to treat TB, malaria, and HIV, to assess how well the humanised mouse matches clinical observations. They found lower drug clearance rates in 8HUM mice than in wild-type animals using both in vitro tests in hepatic microsomes and primary hepatocytes and in vivo tests in live animals, which much more closely matched human drug metabolism. Drug metabolites can be pharmacologically active or can give rise to toxicity, so these issues are important to consider for preclinical testing. In studying metabolite profiles for 14 approved medicines, the team found that 8HUM metabolite profiles were, in many cases, almost identical to profiles in human but very different to those in wild-type mice.
Although this paper focused on drug development for infectious diseases, it is clear that humanised drug metabolism could be beneficial for finding new treatments for many other kinds of diseases, including neurodegeneration, cardiovascular disease, and cancer. Indeed, our first set of projects receiving support from the Director’s Fund includes a project to evaluate the use of the 8HUM model for anticancer drug development. This project, led by Roland Wolf, Karen Blyth, co-lead of the Cancer Cluster, and Kevin Read, the corresponding author on this paper, aims to establish the utility of the 8HUM mouse with clinically relevant mouse models of colon and skin cancers available through the Cancer Cluster. In particular, they aim to test and optimise therapies involving drug combinations.
With the pharmacokinetics, metabolite profiles, and drug –drug interactions for 8HUM mice in much closer alignment with clinical observations than those seen for wild-type mice, this humanised mouse model has the potential to accelerate drug discovery for a very wide range of diseases as well as to replace the use of wild-type mice in preclinical drug testing.
Roland Wolf is a founder of PhaSER Biomedical, a spin-out company formed in 2022 to collaborate with multiple companies, universities, and charitable organisations to ensure that the 8HUM mouse model is used as widely as possible in drug discovery and development applications. The startup recently received a $2.3 million grant from the Bill and Melinda Gates Foundation as part of the Global Health Discovery Collaboratory.
Roland: “We are delighted to receive this support from the Gates Foundation, which will allow us to provide these valuable mice to drug discovery groups working on some of the most pressing global healthcare challenges we are faced with today.”